How much does hepatitis C cost under Medicare Part D?
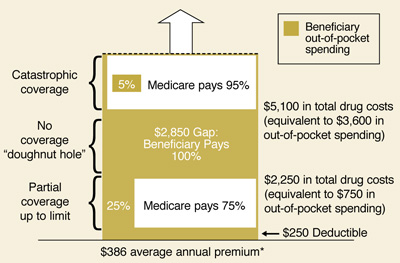
In 2019, Medicare Part D spent approximately $2.5 billion for hepatitis C drugs to treat 50,000 beneficiaries with the disease. Three drugs—Harvoni, Epclusa, and Mavyret—accounted for 93 percent of expenditures, with annual Medicare costs ranging from $28,000 to $77,000 per beneficiary. A portion of these totals was shared by Medicare beneficiaries who faced …
Can drug prices be negotiated for hepatitis C?
See Ornstein Charles, The Cost of a Cure: Medicare Spent $4.5 Billion on New Hepetitis C Drugs Last Year, Propublica (2015), https://www.propublica.org/artide/cost-of-a-cure-medicare-spent-4.5-billion-on-hepatitis-c-drugs-last-year (reviewing the cost of …
How much can hepatitis C Drugs Save You?
1 day ago · Many Medicare beneficiaries without low-income subsidies do not fill high-price specialty drug prescriptions, new research shows. For the four conditions studied — cancer, hepatitis C, immune ...
How long does hepatitis C treatment last?
Hepatitis C drugs are pricey · Harvoni costs $94,500 for a 12-week treatment · Mavyret costs $39,600 for a 12-week treatment · Zepatier costs (1) … Nov 21, 2018 — A 28-day supply costs $22,120, and a 12-week supply costs $66,360.

Does Medicare pay for hep C treatment?
Medicare covers screenings to detect hepatitis C, often at no cost. Medicare Part D plans must include at least one hepatitis C treatment medication. These prescription drugs are often still expensive if you don't have a low-income subsidy to help pay for them.Sep 14, 2020
Does Medicare cover hepatitis?
Hepatitis B Virus (HBV) infection screenings Medicare covers an HBV screening if your primary care doctor orders one and you meet one of these conditions: You're at high risk for HBV infection.
How do you pay for hep C treatment?
Funding Resources Available to Hep C PatientsPharmaceutical Programs. ... The American Liver Foundation (ALF) ... NeedyMeds. ... Help-4-Hep. ... The HealthWell Foundation. ... The Pharmaceutical Research and Manufacturers of America (PhRMA) ... The Patient Access Network (PAN) Foundation. ... The Patient Advocate Foundation.Jun 9, 2021
What is the cost of treating hep C?
The cost of hep C treatment varies depending on the type of drug. However, an 8- to 12-week course can range from $54,000 to $95,000 (or higher). For example, the price of a 12-week course of Zepatier can be as much as $54,600, and a 12-week course of Harvoni can cost as much as $94,500.Sep 2, 2021
Is hep C test covered by insurance?
What about cost? Under the Affordable Care Act, insurance plans must cover hepatitis C testing for certain groups. That means you may be able to get tested at no cost to you.Dec 1, 2015
Does Medicare cover CPT 87340?
A: Yes, according to CMS coverage guidelines. 3 Q: Are the CPT codes 86704, 86706, 87340 and 87341 only for pregnant individuals?Jun 9, 2021
Who qualifies for hep C treatment?
With the exception of pregnant women, the World Health Organization recommends treatment be offered to all individuals aged 12 years or older diagnosed with HCV, regardless of their disease stage.Oct 7, 2019
Will you always test positive for hep C?
A reactive or positive antibody test means you have been infected with the hepatitis C virus at some point in time. Once people have been infected, they will always have antibodies in their blood. This is true if they have cleared the virus, have been cured, or still have the virus in their blood.
How much is sofosbuvir cost?
About Sofosbuvir / Velpatasvir The lowest GoodRx price for the most common version of sofosbuvir / velpatasvir is around $3,639.60, 64% off the average retail price of $10,220.54.
Does United HealthCare cover hep C treatment?
United HealthCare Services Inc. has agreed to expand its coverage of hepatitis C drugs as part of a nationwide class action settlement valued at more than $300 million.
What is the current treatment for hep C?
Hepatitis C is treated using direct-acting antiviral (DAA) tablets. DAA tablets are the safest and most effective medicines for treating hepatitis C. They're highly effective at clearing the infection in more than 90% of people. The tablets are taken for 8 to 12 weeks.
What is the latest treatment for hep C?
The new hepatitis C treatments are sofosbuvir with ledipasvir (Harvoni); sofosbuvir (Sovaldi); daclatasvir (Daklinza); and ribavirin (Ibavyr). These new treatments are now available on the Pharmaceuticals Benefits Scheme.Mar 1, 2016
Who is eligible for 340B?
A hospital that is private, non-profit with a contract with a state or local government to provide health care services to low income individuals who are not entitled to benefits under Medicare or eligible for State Medicaid, is eligible for the 340B Program.
What is a PVP program?
The PVP is a voluntary program for 340B covered entities ...
Can 340B be used for Medicaid?
340B drugs may not be used for Medicaid FFS patients at a contract pharmacy, absent an arrangement between the contract pharmacy, covered entity, and state Medicaid agency to prevent duplicate discounts. Any such arrangement shall be reported to the HRSA Office of Pharmacy Affairs by the covered entity.
Can a covered entity purchase 340B drugs?
The covered entity may purchase and dispense any 340B drugs associated with a service for which the covered entity is responsible, including contraceptives, to that patient, to the extent it aligns with patient definition and is consistent with the scope of the grant. Contract Pharmacy. Are 340B covered entities required to contract ...
What is the HCV bus?
This strategy is part of a national program to educate the population about the importance of screening and linkage to care. When a person visits the bus, they are able to get hep C antibody testing to determine if they may have the virus.
What is the HCA in Washington?
The Health Care Authority (HCA) is partnering with the Department of Health (DOH) and AbbVie US LLC, a research-based global biopharmaceutical company, in an effort to eliminate hepatitis C (HCV) in Washington State by 2030. AbbVie was awarded the state contract because they provided the best overall portfolio and offer a product ...
What is the most common blood borne disease in the United States?
Hepatitis C (HCV) is the most common blood borne disease in the United States. Between 75 and 85 percent of people infected with HCV develop chronic HCV. Chronic HCV is a lifelong virus that can cause severe scarring (cirrhosis) of the liver, liver cancer, the need for a liver transplant, and even death.
Is HCV curable?
HCV is curable and elimination is possible. Elimination is a state where HCV is no longer a public health threat, and where those few who become infected quickly learn their status and receive curative treatment, preventing the forward spread of the virus.