
How do I become a Medicare DME provider?
3 Steps to Becoming DME Supplier for Medicare
- Acquire a National Providers Identification (NPI) Number If you don’t have one already, you won’t meet the DME license requirements. ...
- Complete Your Medicare Enrollment Application You will need to complete the enrollment application using the PECOS’s online system through their website here.
- Work with Your Medicare Administrative Contractor (MAC)
What DME does Medicare pay for?
What durable medical equipment does Medicare cover? Medicare covers a range of items, supplies and equipment such as durable medical equipment. The list of DME that is covered by Medicare includes (but is not limited to): Air-fluidized beds and various other support surfaces Blood pressure monitors Blood sugar monitors Blood sugar test strips Canes
What does DMEPOS stand for?
What does DMEPOS stand for? DMEPOS stands for Durable Medical Equipment, Prosthetics , Orthotics, and Supplies. This definition appears very frequently and is found in the following Acronym Finder categories: Science, medicine, engineering, etc. Organizations, NGOs, schools, universities, etc.
How to become an authorized Medicare DME supplier?
- Obtain DMEPOS accreditation from a CMS-approved organization
- Enroll in the Medicare program as a DMEPOS Supplier
- Post a surety bond to the National Supplier Clearinghouse (NSC)
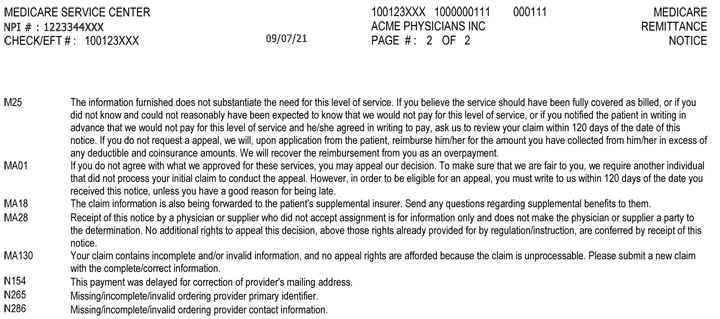
What does Dmepos stand for in Medicare?
DMEPOS stands for durable medical equipment, prosthetics, orthotics and supplies. Page 2. DMEPOS supplier means an entity or individual, including a physician or a Part A provider, which sells or rents Part B covered items to Medicare beneficiaries and which meets the standards in paragraphs (c) and (d) of this section ...
What is included in Dmepos?
Current DMEPOS issues include: Competitive Bidding....DMEPOS = Durable Medical Equipment, Prosthetics, Orthotics and Supplies.Can withstand repeated use.Is used primarily and customarily to serve a medical purpose.Generally is not useful to a person in the absence of illness or injury.Is appropriate for use in the home.
What is Dmepos accreditation?
Page 1. DMEPOS ACCREDITATION. FACT SHEET. Section 302 of the Medicare Modernization Act (the Act) required the Secretary to establish and implement quality standards for suppliers of Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS).
Where are Dmepos claims submitted to?
supply patients with durable medical equipment (DME) (e.g., canes, crutches); DMEPOS claims are submitted to DME Medicare administrative contractors (MACs) who are awarded contracts by CMS; each DME MAC covers a specific geographic region of the country and is responsible for processing DMEPOS claims for its specific ...
Are shower chairs covered by Medi Cal?
No, Medicare does not cover shower chairs, because they're not considered medically necessary.
Does Medi cal cover CPAP?
Medi-Cal covers CPAP equipment for patients ages 18 and older who meet the established criteria for obstructive sleep apnea (OSA) or who have another medical condition(s) for which CPAP equipment is medically necessary.
What are non accredited products?
Non-accredited products are products provided by a DMEPOS supplier that do not require the DMEPOS supplier to obtain accreditation from a CMS approved agency in order for the DMEPOS supplier to bill Medicare for that product.
What is a surety bond for Medicare?
These bonds help protect against billing fraud and abuse. Typically, Medicare surety bonds renew annually and stay in place as long as you keep your license. The annual premium is often only 0.5% of the bond amount ($250) but may be higher in some circumstances.
How much does Achc accreditation cost?
ACHC - When you enroll a $1,500.00 not refundable deposit is required, $300.00 of which represents ACHC's administrative fee. You will then be sent a contract and 30 days after a payment of $6,900 is due, representing the remainder of your contract.
What are DME products?
Equipment and supplies ordered by a health care provider for everyday or extended use. Coverage for DME may include: oxygen equipment, wheelchairs, crutches or blood testing strips for diabetics.
Are L codes considered DME?
L-Codes: Splinting and Bracing Before you can bill L-codes to Medicare, you must be a certified DME provider. If you haven't received your DME certification yet, here are some tips for billing Medicare for orthotic services: Bill 97760 for the initial assessment; Bill the patient for the device or supplies; and.
Is a cast considered DME?
Comfort, Non-Therapeutic. Comfort, non-therapeutic cast-braces are considered medically necessary DME after a fracture or surgery.
What is DMEPOS in Medicare?
Suppliers who receive Medicare reimbursement for durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS) are required to: If your business doesn’t dispense or furnish DMEPOS, you should use the Medicare Enrollment Guide for Providers & Suppliers.
What to do if your business doesn't dispense DMEPOS?
If your business doesn’t dispense or furnish DMEPOS, you should use the Medicare Enrollment Guide for Providers & Suppliers. If you’re enrolling a hospital, critical care facility, skilled nursing facility, home health agency, hospice, or other similar institution, you should refer to the Medicare Enrollment Guide for Institutional Providers.
How much is PECOS 2020?
Complete the online PECOS application. The Medicare Application Fee for 2020 is $595. You can pay the fee on the PECOS Medicare Fee Payment page.
What is DME in Medicare?
Medicare payment for durable medical equipment (DME), prosthetics and orthotics (P&O), parenteral and enteral nutrition (PEN), surgical dressings, and therapeutic shoes and inserts is equal to 80 percent of the lower of either the actual charge for the item or the fee schedule amount calculated for the item, less any unmet deductible.
When did the DME and P&O fee schedules start?
OBRA of 1990 added a separate subsection, 1834 (h), for P&O. The DME and P&O fee schedules were implemented on January 1, 1989 with the exception of the oxygen fee schedules, which were implemented on June 1, 1989. Section 13544 of OBRA of 1993, which added section 1834 (i) to the Social Security Act, mandates a fee schedule for surgical dressings;
When was the CMS meeting in 2012?
Monday, July 23; 9am-1pm ET. CMS hosted a public meeting on July 23, 2012 that provided an opportunity for consultation with representatives of suppliers and other interested parties regarding options to adjust the Medicare payment amounts for non mail order diabetic testing supplies.
What is the MLR for DME?
This rule also proposes the implementation of budget-neutral fee schedules for splints and casts, and intraocular lenses (IOLs) inserted in a physician’s office. Finally, this rule would make a few technical amendments and corrections to existing regulations related to payment for DMEPOS items and services in the End-Stage Renal Disease Prospective Payment System Proposed Rulemaking. View CMS-1526-P .
When will CMS increase fee schedule?
On May 11, 2108, CMS published an interim final rule with comment period (IFC) that increases the fee schedule rates for items furnished from June 1, 2018, through December 31, 2018, for certain durable medical equipment (DME) and enteral nutrition furnished in rural and non-contiguous areas of the country not subject to the Durable Medical Equipment, Prosthetics, Orthotics and Supplies (DMEPOS) Competitive Bidding Program (CBP). To safeguard beneficiary access to necessary items and services, this rule increases the fee schedule amounts for certain DME and enteral nutrition in rural and noncontiguous areas to a blend of 50 percent of the fee schedule amounts that would have been paid from June 1, 2018, through December 31, 2018, had no adjustments been made and 50 percent of the adjusted fee schedule amounts. For areas other than rural or non-contiguous areas, the fee schedules for certain DME and enteral nutrition codes will continue to be based on 100 percent of the adjusted fee schedule amounts from June 1, 2018 through December 31, 2018.
When was the CMS Pen rule published?
Corrections were published on December 28, 2018 in CMS-1691-CN. This rule established a methodology for adjusting fee schedule amounts for certain items using information from ...
When was the Medicare non-mail order for diabetic testing supplies passed?
Diabetic Testing Supplies Provisions of the American Taxpayer Relief Act of 2012. On Wednesday, January 2, 2013, the President signed into law the American Taxpayer Relief Act of 2012 . Section 636 of this new law revises the Medicare non-mail order fee schedule amounts for diabetic testing supplies.
What is a DME?
Durable Medical Equipment (DME) Durable Medical Equipment (DME) is equipment which: Can withstand repeated use, Is primarily and customarily used to serve a medical purpose, Generally is not useful to a person in the absence of an illness or injury, and. Is appropriate for use in the home.
What supplies are covered by DME?
Supplies and accessories that are necessary for the effective use of medically necessary DME are covered. Supplies may include drugs and biologicals that must be put directly into the equipment in order to achieve the therapeutic benefit of the DME or to assure the proper functioning of the equipment.
What is covered under the aphakia benefit?
Coverage under this benefit includes, but is not limited to, artificial arms and legs, breast prostheses, eye prostheses, parenteral and enteral nutrition, ostomy supplies, urological supplies in beneficiaries with permanent urinary incontinence, and glasses or contact lenses in beneficiaries with aphakia or pseudophakia.
What is Medicare Part B?
Medicare Part B covered services processed by the DME MAC fall into the following benefit categories specified in Section 1861 (s) of the Social Security Act: A brace is a rigid or semi-rigid device used for the purpose of supporting a weak or deformed body member or restricting or eliminating motion in a diseased or injured part of the body.
Is a transplant covered by Medicare?
Immuno-suppressive Drugs. Immunosuppressive drugs are covered if the transplant met the Medicare covered criteria in effect at the time of the transplant, the beneficiary was enrolled in Medicare Part A at the time of the transplant and the beneficiary is enrolled in Medicare Part B at the time the drugs are dispensed.
Is immunosuppressive therapy covered by DME?
Immunosuppressive drugs used for indications other than transplantation are not to be billed to the DME MAC. Supplies used in conjunction with parenterally administered immunosuppressive drugs are not covered under this benefit category. Oral Anticancer Drugs.
Consolidated DMEPOS Lists
The Master List (PDF) is a library of all DMEPOS items posing vulnerabilities to the Trust Fund that may require providers/suppliers to comply with additional conditions related to payment requirements. From this list, items may be selected for one or both of the Required Lists:
Required Face-to-Face Encounter and Written Order Prior to Delivery List
Review contractors assess compliance with the face-to-face encounter and written order prior to delivery requirements. Some items (such as PMDs) have statutorily imposed requirements.
Written Order Prior to Delivery (WOPD) Requirements
For items on the Required Face-to-Face Encounter and Written Order Prior to Delivery List, a complete order is required prior to the item’s delivery.
Face-to-Face Encounter requirements applicable to certain DMEPOS items
For all items requiring a face-to-face encounter, a practitioner visit is required within six months preceding the order. Note: face-to-face encounters for PMDs were previously required within 45 days preceding the written order.
Learn More
DMEPOS Written Order, Face-to-Face Encounter, and/or Prior Authorization Requirements (PDF): Learn more about the standard elements for a DMEPOS order and items potentially subject to face-to-face encounter and written order prior to delivery and/or prior authorization requirements.
