
Medicare Part D
Medicare Part D
Medicare Part D, also called the Medicare prescription drug benefit, is an optional United States federal-government program to help Medicare beneficiaries pay for self-administered prescription drugs through prescription drug insurance premiums. Part D was originally propo…
How to find the best Medicare Part D drug plan?
Specialty tier drugs represent a limited number of Part D drugs that are used by a small proportion of beneficiaries. The current specialty tier threshold of $600 per month was established in 2008 and has remained at that level based on Part D data analysis.
Who sells Medicare Part D?
Jan 15, 2021 · Under the final rule, beginning January 1, 2022, CMS is allowing Part D plans to have a second, “preferred” specialty tier with a lower cost sharing amount than their other specialty tier. This change is designed to give Part D plans more tools to negotiate better deals with manufacturers and lower out-of-pocket costs for enrollees in exchange for placing those …
What is a Tier 5 specialty tier drug?
May 14, 2021 · Quick Summary: Allows Medicare Advantage and Part D plans to offer a second specialty tier. Requires that if there are two specialty tiers, one must be a “preferred” tier that offers lower cost-sharing than the maximum allowable cost-sharing. Requires plan sponsors to permit tiering exceptions between the two specialty tiers.
What is a Medicare Part D preferred pharmacy?
Jan 18, 2022 · For example, the drugs on Tier 1 in a four-tier system typically cost between $10 and $25. However, the Tier 1 medications on a five- or six-tier formulary are more likely to cost $3 to $5. Below is an example of pricing and prescriptions included in a five-tier formulary. Many plans charge a flat co-pay for drugs on lower tiers and a ...
What is specialty tier?
What is a Tier 5 specialty drug?
What are Speciality tier drugs?
What are the 4 phases of Part D coverage?
Is there an out of pocket maximum for Medicare Part D?
How do you determine a drug tier?
What is a Tier exception?
What tier is gabapentin?
Is Symbicort covered by Medicare Part D?
How does Part D Medicare work?
Does Medicare Part D have a copay?
Typically, you will be responsible for a copayment if you have a Medicare Advantage or Part D prescription drug plan. And you will have to pay coinsurance if you have Original Medicare — Medicare Part A and Part B.
Do all Medicare Part D plans have a deductible?
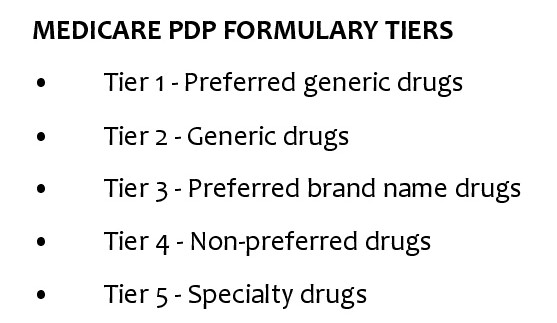
What are the tiers of Medicare Part D?
The Medicare Part D tiers refer to how drugs are organized in a formulary. They include both generic and brand name drugs, covered for different prices. Most commonly there are tiers 1-5, with 1 covering the lowest-cost drugs and 5 covering the most expensive specialty medications.
Does Medicare Part D have a deductible?
Medicare Part D tiers 1 and 2 are often set up to exempt you from paying a deductible, whereas with drugs in the higher tiers you may have to pay the full drug cost until you meet the deductible, then pay a copay/coinsurance.
What are the tiers of a drug?
They include both generic and brand name drugs, covered for different prices. Most commonly there are tiers 1-5, with 1 covering the lowest-cost drugs and 5 covering the most expensive specialty medications.
What is preferred drug?
Preferred drugs means a certain set of types of medications that have been approved by the insurance company to be in this low-cost grouping. Generic refers to non-name brand versions of each type of drug.
When will CMS allow Part D plans to have a second specialty tier?
Under the final rule, beginning January 1, 2022, CMS is allowing Part D plans to have a second, “preferred” specialty tier with a lower cost sharing amount than their other specialty tier. This change is designed to give Part D plans more tools to negotiate better deals with manufacturers and lower out-of-pocket costs for enrollees in exchange for placing those products on the “preferred” specialty tier.
When will Part D start?
The final rule will require Part D plans to offer real-time comparison tools to enrollees starting January 1, 2023, so enrollees have access to real-time formulary and benefit information, including cost-sharing, to shop for lower-cost alternative therapies under their prescription drug benefit plan. Enrollees would be able to compare cost sharing ...
What is CMS contract year 2021?
In the June 2, 2020 Federal Register, the Centers for Medicare & Medicaid Services (CMS) issued the Contract Year 2021 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, and Medicare Cost Plan Program final rule (85 FR 33796) that implemented a subset of the proposals from the February 2020 proposed rule (85 FR 9002). That final rule focused on more immediate regulatory actions and was primarily intended to implement certain changes before the contract year 2021, stemming from the Bipartisan Budget Act of 2018 (BBA of 2018) and the 21st Century Cures Act (Cures Act). That final rule also codified several existing CMS policies and implemented other technical changes.
What is the final rule for Part D?
The final rule will require Part D plans to offer real-time comparison tools to enrollees starting January 1, 2023, so enrollees have access to real-time formulary and benefit information, including cost-sharing, to shop for lower-cost alternative therapies under their prescription drug benefit plan. Enrollees would be able to compare cost sharing to find the most cost-effective prescription drugs for their health needs. For example, if a doctor recommends a specific cholesterol-lowering drug, the enrollee could look up what the copay would be and see if a different, similarly effective option might save the enrollee money. With this tool, enrollees will be better able to know what they’ll need to pay before they’re standing at the pharmacy cash counter.
Do pharmacy plans have to disclose performance measures?
Under the Part D program, plans currently do not have to disclose to CMS the measures they use to evaluate pharmacy performance in their network agreements. CMS has heard concerns from pharmacies that the measures plans use to assess their performance are unattainable or otherwise unfair. The measures used by plans potentially impact pharmacy reimbursements. Therefore, starting January 1, 2022, CMS is requiring Part D plans to disclose pharmacy performance measures to CMS, which will enable CMS to better understand how such measures are applied. CMS will also be able to report pharmacy performance measures publicly to increase transparency on the process and to inform the industry in its new efforts to develop a standard set of pharmacy performance measures.
What is CMS finalizing?
CMS is finalizing a number of provisions that will reduce the administrative burden for PACE organizations related to the service determination request process and improve participants’ care and experience, including the participant appeals process and participant rights, and strengthen requirements related to the provision of services and record keeping.
What is a Part D specialty tier?
Under the final rule, Part D plans may establish a second specialty tier, splitting specialty drugs between a non-preferred specialty tier and a preferred specialty tier, with the preferred tier carrying lower cost-sharing obligations than the non-preferred tier. By allowing plans to apply differential co-insurance obligations to specialty drugs, this dual specialty tier model is designed to give plans flexibility to incentivize beneficiaries to select lower-cost specialty drugs.
Do specialty tiers have tiering exceptions?
Presently, specialty tier medications are not required to have a tiering exception from the specialty tier down to a “lower” non -specialty tier. Plans managing their formularies (every Part D plan) must maintain a benefit design actuarially equivalent to the Defined Standard and allowing a non -specialty tier exception “would likely increase costs elsewhere such as increased cost-sharing on generic drug tiers or increases in premiums.” In the final rule, CMS formally clarified that Part D sponsors may design their exception processes so that Part D drugs on the specialty tier (s) are not eligible for a tiering exception to non- specialty tiers to maintain actuarial equivalence.
When is the final rule for the CY 2022?
With the bids due by June 2021 and the expiration of the 60-day regulatory delay period, the final rule is expected to remain unchanged for CY 2022.
What is Medicare Part D?
Key Takeaways. Medicare Part D is an optional coverage available for a cost that can help pay for prescription drugs. Medicare Part D is sold by private insurance companies that have contracted with Medicare to offer it to people eligible for Medicare. Not all Part D plans operate everywhere, nor do all of the plans offer ...
What are the different tiers of Medicare?
The drugs in the plan’s formulary may be further placed into different tiers that determine your cost. For example: 1 Tier 1: The most generic drugs with the lowest copayments 2 Tier 2: Preferred brand-name drugs with medium copayments 3 Tier 3: Non-preferred brand name drugs with higher copayments 4 Specialty: Drugs that cost more than $670 per month, the highest copayments 4
What drugs are covered by Part D?
Drugs covered by each Part D plan are listed in their “formulary,” and each formulary is generally required to include drugs in six categories or protected classes: antidepressants, antipsychotics, anticonvulsants, immunosuppressants for treatment of transplant rejection, antiretrovirals, and antineoplastics.
What happens if you don't have Part D coverage?
The late enrollment penalty permanently increases your Part D premium. 3. Prescription drug coverage that pays at least ...
How long can you go without Medicare Part D?
You can terminate Part D coverage during the annual enrollment period, but if you go 63 or more days in a row without creditable prescription coverage, you’ll likely face a penalty if you later wish to re-enroll. To disenroll from Part D, you can: Call Medicare at 1-800-MEDICARE.
How to disenroll from Medicare?
Call Medicare at 1-800-MEDICARE. Mail or fax a letter to Medicare telling them that you want to disenroll. If available, end your plan online. Call the Part D plan directly; the issuer will probably request that you sign and return certain forms.
What happens if you don't enroll in Part D?
Not enrolling in Part D during the initial enrollment period could result in a late-enrollment penalty that permanently increases your Part D premium.
How much does Medicare Part D cost?
Specialty tier drugs—defined by Medicare as drugs that cost more than $670 per month in 2019—are a particular concern for Part D enrollees in this context.
Does Medicare Part D cover out-of-pocket costs?
Medicare Part D has helped to make prescription drugs more affordable for people with Medicare, yet many beneficiaries continue to face high out-of-pocket costs for their medications.
Summary
Recent regulatory action released in the final days of the Trump administration related to Medicare Advantage (MA) and Part D could significantly impact plan and manufacturer calendar year (CY) 2022 contracting strategies and stakeholder advocacy priorities.
Specialty Tier Changes
On January 15, the CMS finalized the CY 2022 MA and Part D rule, which includes a provision that will allow Part D plans to implement a second, preferred specialty tier with lower cost sharing beginning in CY 2022.
CY 2022 Center for Medicare and Medicaid Innovation (CMMI) Demonstrations
On January 14 and 19, the CMMI released the CY 2022 Request for Applications (RFAs) for the Part D Senior Savings Model and the Part D Modernization Model, respectively.
Move to Point-of-Sale (POS) Discounts
Along with these recent regulatory actions, stakeholders will need to consider implementation of the Office of Inspector General final Anti-Kickback Statute (AKS) rebate rule released in November 2020.
Future Outlook
On January 20, the Biden administration released a memo directing executive departments and agencies to review any regulations that have not yet taken effect and to consider re-opening a 30-day public comment period. This may provide stakeholders an additional opportunity to engage with the new administration on these policy changes.
