How is the TMS treatment order written?
• The TMS treatment order is written by a psychiatrist (MD or DO) who has examined the individual and reviewed the record.
Is TMS treatment FDA approved?
• TMS treatment is provided using a device that is approved by the U.S. Food and Drug Administration (FDA) for the treatment of major depressive disorder (CMS L36469, 2021; L33398; L34641; L34869; 2020; L34522; L34998; L37086; L37088, 2019).
What is transcranial magnetic stimulation (TMS)?
Transcranial magnetic stimulation (TMS) is a noninvasive method of brain stimulation. The technique involves placement of a small coil over the scalp and passing a rapidly alternating current through the coil wire which produces a magnetic field that passes unimpeded through the brain.

What is a TMS code?
CPT Code 90868 Therapeutic repetitive transcranial magnetic stimulation (TMS) treatment; subsequent delivery and management, per session.
How do I bill for TMS?
CPT 90869 Therapeutic repetitive transcranial magnetic stimulation (TMS) treatment; subsequent motor threshold re-determination with delivery and management, is considered reasonable and necessary when there is a change in clinical status or medical regimen that is expected to alter cortical excitability.
What is the CPT code 90867?
Code. Description. 90867. THERAPEUTIC REPETITIVE TRANSCRANIAL MAGNETIC STIMULATION (TMS) TREATMENT; INITIAL, INCLUDING CORTICAL MAPPING, MOTOR THRESHOLD DETERMINATION, DELIVERY AND MANAGEMENT.
Is TMS the same as DBS?
What's the Difference Between TMS and Deep Brain Stimulation? TMS uses noninvasive magnetic stimulation to safely activate and strengthen the brain's communication channels. Deep brain stimulation, on the other hand, is a surgical procedure where electrodes are planted directly onto the brain.
What does CPT code 90833 mean?
psychotherapy+90833 - Use add-on code for Individual psychotherapy, insight oriented, behavior modifying and/or supportive, 30 minutes with the patient and/or family member (time range 16-37 minutes), when performed with an evaluation and management service.
Who can bill CPT code 90837?
CPT code 90837 doesn't have to be billed by a licensed medical doctor. It's mainly used by licensed mental health professionals. Typically, Licensed Clinical Social Workers, Licensed Professional Clinical Counselors, Licensed Marriage and Family Therapists, and Clinical Psychologists will bill CPT code 90837.
What is CPT code G2083?
HCPCS code G2083 for Office or other outpatient visit for the evaluation and management of an established patient that requires the supervision of a physician or other qualified health care professional and provision of greater than 56 mg esketamine nasal self-administration, includes 2 hours post-administration ...
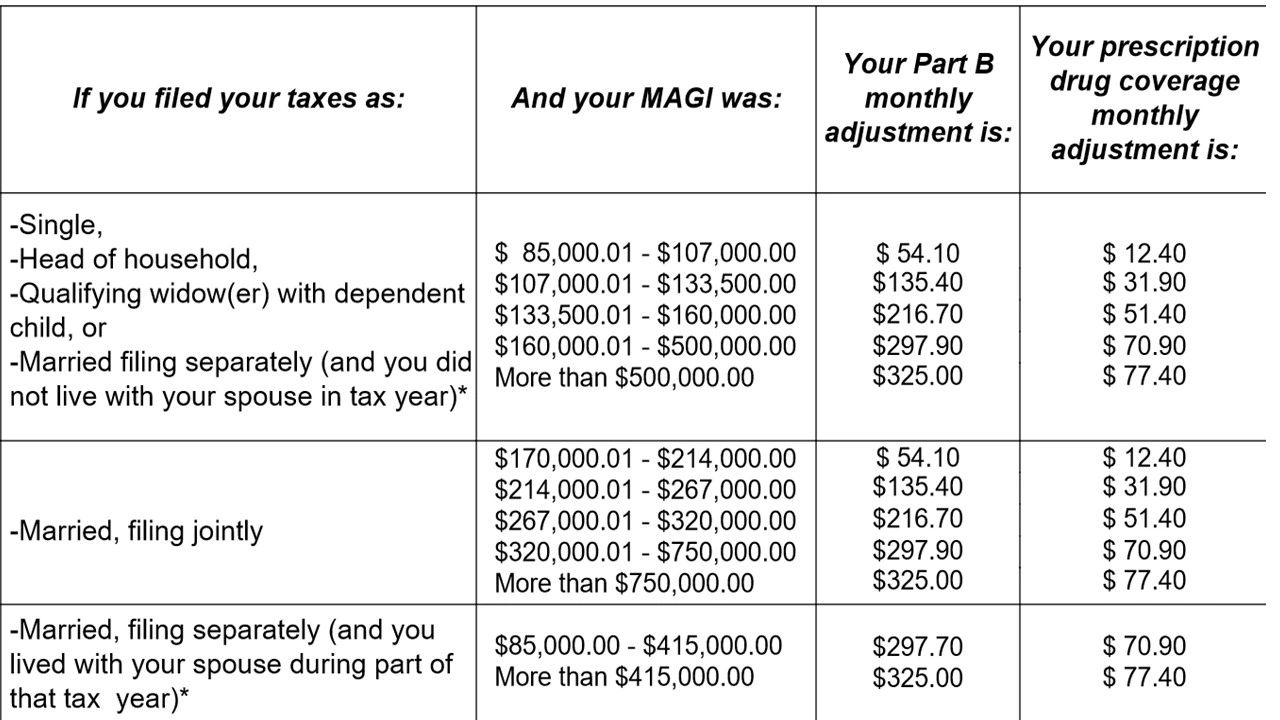
Does Medicare cover maintenance TMS?
If you're 65 years or older and suffer from depression, you may have wondered, “Does Medicare cover TMS?” Medicare does in fact cover transcranial magnetic stimulation (TMS) treatments.
What is the CPT code 90686?
Flu Shot CodingAdministration & Diagnosis CodesVaccine Codes & Descriptors90685Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, 0.25 mL dosage, for intramuscular use90686Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, 0.5 mL dosage, for intramuscular use25 more rows•Sep 14, 2021
What is the difference between TMS and deep TMS?
While Traditional rTMS has a narrower range of activation, Deep TMS manages to safely reach deeper brain structures directly, which contributes to its higher level of efficacy. Various studies have shown that both Deep TMS and Traditional rTMS are safe and effective courses of treatment.
Is TMS deep brain stimulation?
Risks. Repetitive TMS is a noninvasive form of brain stimulation used for depression. Unlike vagus nerve stimulation or deep brain stimulation, rTMS does not require surgery or implantation of electrodes. And, unlike electroconvulsive therapy (ECT), rTMS doesn't cause seizures or require sedation with anesthesia.
What is the difference between TMS and neurofeedback?
TMS and neurofeedback are completely different methods employed to change brain activity. How are TMS and neurofeedback different? TMS uses magnets and is primarily applied to treat depression. Neurofeedback employs feedback to help you learn to stimulate or change your brain.
What is CMS in healthcare?
The Centers for Medicare & Medicaid Services (CMS), the federal agency responsible for administration of the Medicare, Medicaid and the State Children's Health Insurance Programs, contracts with certain organizations to assist in the administration of the Medicare program. Medicare contractors are required to develop and disseminate Local Coverage Determinations (LCDs). CMS believes that the Internet is an effective method to share LCDs that Medicare contractors develop. While every effort has been made to provide accurate and complete information, CMS does not guarantee that there are no errors in the information displayed on this web site. THE UNITED STATES GOVERNMENT AND ITS EMPLOYEES ARE NOT LIABLE FOR ANY ERRORS, OMISSIONS, OR OTHER INACCURACIES IN THE INFORMATION, PRODUCT, OR PROCESSES DISCLOSED HEREIN. Neither the United States Government nor its employees represent that use of such information, product, or processes will not infringe on privately owned rights. In no event shall CMS be liable for direct, indirect, special, incidental, or consequential damages arising out of the use of such information, product, or process.
What brain areas are affected by rTMS?
Twenty studies met inclusion criteria with 19 using rTMS and one dTMS. All but one of the rTMS trials are included in the meta-analyses described above. Included brain areas were the dorsolateral prefrontal cortex (DLPFC), supplementary motor area (SMA), orbitofrontal/medial prefrontal cortex (OFC), and anterior cingulate cortex (ACC). Frequency stimulation was low (1 Hz) or high (>/=5 Hz). Treatment duration varied from two to six weeks with follow-up ranging from none to three months. Three tables listed 16 of the studies. Nine had Y-BOCS score reductions with rTMS versus sham; eight showed no significant difference. Summaries of dTMS studies follow. The authors concluded treatment of OCD with neurostimulation shows promise, but it is yet to be determined how best to optimize the approach using rTMS or dTMS to achieve clinically relevant results.
Is rtms FDA approved?
The rTMS treatment is delivered by a device that is FDA-approved or –cleared for the treatment of MDD in a safe and effective manner. rTMS treatment should generally follow the protocol and parameters specified in the manufacturer’s user manual, with modifications only as supported by the published scientific evidence base; and
Is rTMS a less invasive treatment?
History of response to electroconvulsive therapy (ECT) in a previous or current MDD episode, or inability to tolerate ECT, and rTMS is considered a less invasive treatment option; and
Who writes the order for treatment?
The order for treatment (or retreatment) is written by a physician (MD or DO) who has examined the patient and reviewed the record. The physician must have experience in administering rTMS therapy and the treatment must be given under direct supervision of this physician, i.e., he or she must be in the area and be immediately available.
Is CPT a year 2000?
CPT is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to, the implied warranties of merchantability and fitness for a particular purpose. AMA warrants that due to the nature of CPT, it does not manipulate or process dates, therefore there is no Year 2000 issue with CPT. AMA disclaims responsibility for any errors in CPT that may arise as a result of CPT being used in conjunction with any software and/or hardware system that is not Year 2000 compliant. No fee schedules, basic unit, relative values or related listings are included in CPT. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this file/product is with CMS and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this file/product. This Agreement will terminate upon no upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Can you use CPT in Medicare?
You, your employees and agents are authorized to use CPT only as contained in the following authorized materials of CMS internally within your organization within the United States for the sole use by yourself, employees and agents. Use is limited to use in Medicare, Medicaid or other programs administered by the Centers for Medicare and Medicaid Services (CMS). You agree to take all necessary steps to insure that your employees and agents abide by the terms of this agreement.
What is TMS therapy?
A section of the updated guidelines (Section IV) relates to neurostimulation therapies, including TMS, electroconvulsive therapy (ECT), vagus nerve stimulation (VNS), and deep brain stimulation (DBS), for treating MDD in adults. The subsection for TMS notes that in 2002, Canada approved the use of TMS for treating depressed adults who fail to respond to at least one antidepressant drug. Most available evidence pertains to the use of high-frequency left-sided TMS (HFL-TMS) for this indication. However, direct comparisons among the many open-label studies and randomized controlled studies are hampered by variations in study design and stimulation parameters. Based on available data, the CANMAT recommended that, when using TMS for treatment-resistant MDD, the first TMS approach should be HFL-TMS and the treatment duration should be 30 sessions (3 weeks) instead of 20 sessions (2 weeks). The CANMAT noted that there was minimal evidence regarding the use of TMS for maintaining response/preventing relapse and drew no conclusions regarding TMS for this indication. 5
What is the only magnetic stimulator system approved by the FDA for treating depression?
The only magnetic stimulator system approved by the FDA for treating depression is the NeuroStar® TMS Therapy System (Neuronetics Inc.). This system (product code, OBP) was approved in 2008 for treating adult patients with major depressive disorder (MDD) only when the affected patient has failed to attain satisfactory improvement from at least one antidepressant medication administered in the current depressive episode at or above the minimal effective dose for at least the minimal effective duration and only when TMS is prescribed by and performed under the supervision of a licensed psychiatrist. 4
Does TMS show efficacy?
recent TEC report (2009) evaluated transcranial magnetic stimulation for the treatment of depression and summarized that the randomized clinical trial of TMS does not show definitive evidence of efficacy for its primary endpoint at 4 weeks. Not all outcomes show efficacy, and the analysis is sensitive to alternative methods of analysis. Another limitation of this and other studies of TMS is lack of rigorous evaluation beyond the period of treatment. Although short-term studies are consistent with changes in depression scores due to TMS, the clinical significance and durability of the effect are not well characterized. One metaanalysis indicated no difference in effect between patients with treatment-resistant and nontreatment- resistant depression. The randomized, clinical trial showed a greater effect in patients with only one prior treatment failure, with possibly minimal or no effect in patients with greater than one prior treatment failure. The TEC report indicated that the available evidence does not permit conclusions regarding the effect of TMS on health outcomes or compared with alternatives and it has not yet been demonstrated whether TMS improves health outcomes in the investigational setting. 13
Is TMS considered an investigational treatment?
Transcranial magnetic stimulation (TMS) for the treatment of major depression, migraine headaches, or any other diagnosis is considered investigational and unproven as there is insufficient evidence in the peer reviewed scientific literature on whether the effect of TMS improves health outcomes as compared with alternatives.
Is transcranial magnetic stimulation a treatment option?
In the Practice Guideline for the Treatment of Major Depressive Disorder published 2010, transcranial magnetic stimulation is mentioned as a treatment option for patients who do not respond to pharmacotherapy. The guidelines outline the following key points 6:
How does prior authorization work?
Prior authorization works by having your health care provider or supplier submit a prior authorization form to their Medicare Administrator Contractor (MAC). They must then wait to receive a decision before they can perform the Medicare services in question or prescribe the prescription drug being considered.
Do you have to file a claim for Medicare?
In some instances, you may have to file a Medicare claim for care that you already received. This ensures that your health care provider is properly reimbursed and that you aren’t charged for more out-of-pocket Medicare costs than you actually owe.
Does Medicare have prior authorization?
Medicare coverage contains a lot of qualifiers, exceptions and other criteria. Prior authorization addresses much of the same information required for submitting Medicare claims or filing Medicare appeals, but it does so earlier in the process before the services are provided.
Does Medicare Advantage require prior authorization?
Medicare Advantage plans may sometimes require prior authorization for things like non-emergency hospital care outside of your plan provider network, visiting specialists and some other services.
Do you need prior authorization for Medicare Part C?
It’s not uncommon, however, for beneficiaries of Medicare Advantage (Medicare Part C) plans and Medicare Part D Prescription Drug plans to need prior authorization before receiving some types of care. Prior authorization is most common for getting certain prescription drugs covered by your plan.
How does TMS work?
Transcranial Magnetic Stimulation (TMS) is a non-invasive technique using a device that has been approved by the Food and Drug Administration (FDA) to apply brief magnetic pulses to the brain for the treatment of major depressive disorder. The pulses are administered by passing currents through an electromagnetic coil placed adjacent to the individual’s scalp. The pulses induce an electrical field in the brain tissue, activating neurons in the targeted brain structure. By stimulating areas of the brain, the goal is to lessen the duration or severity of depressive episodes. TMS is typically applied daily in subjects with major depressive disorder who have failed previous antidepressant trials in the current episode.
What is a consensus recommendation for rTMS?
Consensus recommendations for the application of repetitive transcranial magnetic stimulation (rTMS) were published in 2018 by the National Network of Depression Centers rTMS Task Group and the American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments (McClintock et al., 2018). A total of 118 publications (including 3 RCTs) were included in the consensus statement and were supplemented with expert opinion to achieve consensus recommendations on key issues surrounding the administration of rTMS for major depressive disorder (MDD) in clinical practice settings.
Is TMS better than sham TMS?
The results from a majority of studies, including multicenter randomized controlled trials, support the hypothesis that treatment with TMS is superior to sham TMS for the treatment of major depressive disorder. There is also growing research as to the durability of TMS treatment for this population, though the possible influence of concurrent antidepressant use in many study designs continues to pose a methodological limitation. FDA-approved TMS devices can be administered safely when treatment is provided under proper supervision and with adherence to the appropriate therapy manual. There is a need for conclusive evidence from controlled trials on the benefit of maintenance TMS therapy, such as when compared to maintenance antidepressant use. TMS has not been demonstrated to be equivalent in efficacy when compared to ECT for the treatment of major depressive disorder. Patients who are candidates for ECT and instead receive TMS likely do so because TMS is regarded as less invasive.
How many RTMS sessions are there on Medicare?
From November 1, the Medicare Benefits Schedule (MBS) will cover the prescription and treatment mapping by a psychiatrist, an initial course of up to 35 rTMS treatment sessions, a review of treatment, and a re-treatment of up to 15 sessions, for people over 18 who have been diagnosed with medication-resistant major depressive disorder, a spokesperson from the Department of Health said.
What is a rtms?
rTMS is a non-invasive procedure that uses magnetic fields to stimulate nerve cells in the brain to improve symptoms of depression, according to the Mayo Clinic.
Why does Amy use TMS?
Amy* relies on repetitive Transcranial Magnetic Stimulation (rTMS or TMS) to avoid spiralling into a severe depressive state or having suicidal thoughts.
What did Professor Fitzgerald say about RTMS?
Professor Fitzgerald said it had been frustrating to see rT MS treatment evaluated by standards that were not applied to other equivalent treatments which were funded .
Is RTMS listed on the MBS?
Because rTMS is not yet listed on the MBS, the Department of Health said it did not have the data to know what the cost of treating rTMS patients as inpatients was on the public health system.
Can Amy get rtms?
Under this funding model, existing patients like Amy, who receive rTMS regularly, will miss out and have to continue paying the full cost of treatment or be admitted to hospital to receive it.
Is TMS covered by hospital?
Acute courses of TMS are covered if you are a hospital inpatient, which Amy has been in the past under her private health cover.
What is rTMS in VA?
The VA/DoD Clinical Practice Guidelines for the Management of Major Depressive Disorder (2016) suggests offering treatment with repetitive transcranial magnetic stimulation (rTMS) for treatment during a major depressive episode in patients with treatment-resistant major depressive disorder.
What is a consensus recommendation for rTMS?
Consensus recommendations for the application of repetitive transcranial magnetic stimulation (rTMS) were published in 2018 by the National Network of Depression Centers rTMS Task Group and the American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments (McClintock et al., 2018). A total of 118 publications (including 3 RCTs) from 1990 through 2016 were included in the consensus statement and were supplemented with expert opinion to achieve consensus recommendations on key issues surrounding the administration of rTMS for major depressive disorder (MDD) in clinical practice settings.
Does Medicare have a national coverage determination?
Medicare does not have a National Coverage Determination (NCD). Local Coverage Determinations (LCDs) and Local Coverage Articles (LCAs) exist; see the LCDs and/or LCAs for Repetitive Transcranial Magnetic Stimulation (rTMS) in Adults with Treatment Resistant Major Depressive Disorder and Transcranial Magnetic Stimulation for Major Depressive Disorder.
Is TMS better than sham TMS?
The results from a majority of studies, including multicenter randomized controlled trials, support the hypothesis that treatment with TMS is superior to sham TMS for the treatment of major depressive disorder. There is also growing research as to the durability of TMS treatment for this population, though the possible influence of concurrent antidepressant use in many study designs continues to pose a methodological limitation. FDA-approved TMS devices can be administered safely when treatment is provided under proper supervision and with adherence to the appropriate therapy manual. There is a need for conclusive evidence from controlled trials on the benefit of maintenance TMS therapy, such as when compared to maintenance antidepressant use. TMS has not been demonstrated to be equivalent in efficacy when compared to ECT for the treatment of major depressive disorder. Individuals who are candidates for ECT and instead receive TMS likely do so because TMS is regarded as less invasive.